
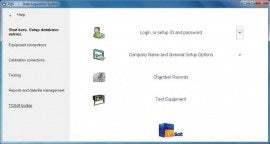
For use with the 2638A and 1586A Data Acquisition System. Compliance with United States Food and Drug Administration (FDA) Title 21 CFR Part 11 regulations on electronic records and signatures for incubation, sterilization, freezing, drying and temperature mapping validation applications in pharmaceutical and biomedical industries.
Model No
TQSOFT-IQ/OQ/D
Condition
New
Manufacturer
Fluke
Hey👋Let's start with your email